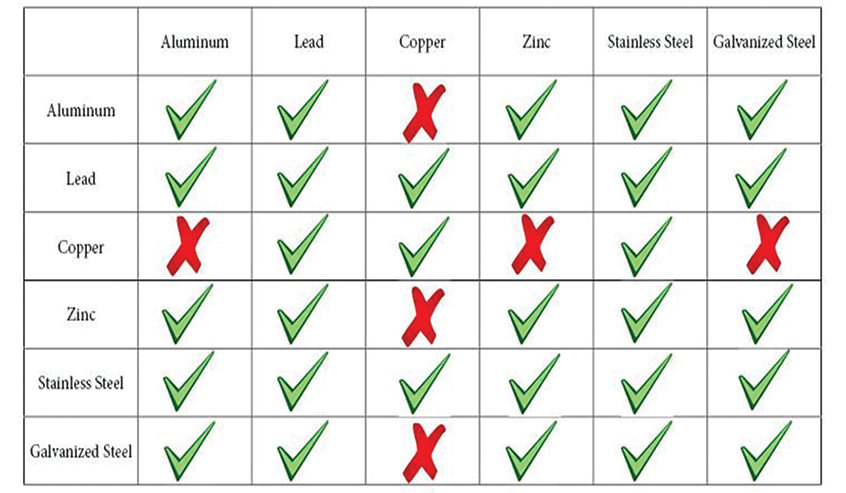
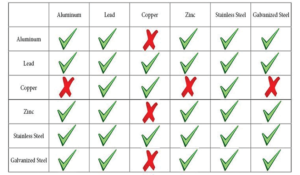
Galvanic Action
Occurs when two electrochemically dissimilar metals are in contact and a conductive path allows electrons and ions to move from one metal to the other creating an electrical current. This current is called “galvanism”.
Galvanic Corrosion
(Also called dissimilar metal corrosion, bimetallic corrosion, electrolytic corrosion or wrongly electrolysis) refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive electrolyte such as a saltwater or acidic solution.
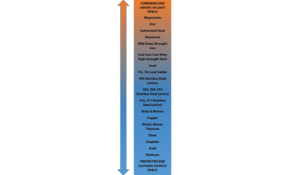
Result
The less noble (anodic) metal experiences accelerated corrosion while the other, more noble (cathodic) metal remains galvanically protected.